-
Your Government
-
- City Council Boards & Commissions Public Meetings & Minutes Municipal Code City News
- Departments Administration Building City Recorder Elections Engineering Finance Human Resources Library
- Departments Municipal Court Parks & Recreation Planning Police Public Works Reservoir Project Urban Renewal Utilities
-
-
Our Community
-
- About St. Helens History of St. HelensState of the CityCourthouse Dock Camera
- Local Events City Calendar Community Day in the Park13 Nights on the RiverIndependence Day Celebration Spirit of HalloweentownRecreation Activities Sand Island CampingKeep It Local CC
- Community Resources City Newsletter City Social Media Emergency Services New Resident InformationProtecting Our Environment
-
-
Business & Development
-
- Local Business Directory Get a Business License City Bids & RFPs Broadband Study
- Business in St. Helens St. Helens Advantages Directions & Transportation Incentives & Financing Resources for Businesses Business Guide Columbia Economic Team Chamber of Commerce
- Current City Projects Waterfront Redevelopment Public Safety Facility Strategic Work Plan
-
-
How Do I?
-
- Apply for a Job Apply for a Committee Find A Park Find COVID Info Find Forms Follow St. Helens - Facebook Follow St. Helens - Twitter Follow St. Helens - YouTube
- Get a Police Report Get a Business License Get a Library Card Get a Building Permit Newsletter Signup Past Public Meetings Pay My Water Bill
- Public Records Request Report a Nuisance Register for Rec Activity Reserve a Park Sign Up for the 911 Alerts Universal Fee Schedule
-
pH - What is it and is this important?
What is pH?
Acidic and basic are two extremes that describe chemicals, just like hot and cold are two extremes that describe temperature. Mixing acids and bases can cancel out their extreme effects; much like mixing hot and cold water can even out the water temperature. A substance that is neither acidic nor basic is neutral.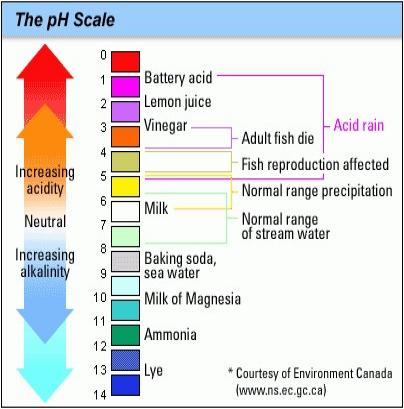
The pH scale measures how acidic or basic a substance is. It ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic. Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times (10 times 10) more acidic than a pH of 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value. For example, a pH of 10 is ten times more alkaline than a pH of 9.
Pure water is neutral, with a pH of 7.0. When chemicals are mixed with water, the mixture can become either acidic or basic. Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are basic.
Chemicals that are very basic or very acidic are called "reactive." These chemicals can cause severe burns. Automobile battery acid is an acidic chemical that is reactive. Automobile batteries contain a stronger form of some of the same acid that is in acid rain. Household drain cleaners often contain lye, a very alkaline chemical that is reactive.
What St. Helens is doing to control pH
The water from the collector wells supplying the St. Helens Filtration plant has a pH value of 6.4 to 6.8, which is considered acidic. Water with a pH value in this range causes corrosion on our plumbing fixtures in our homes and is the chief cause for seeing a “blue-green” type of stain in our sinks and toilet bowls. Also when the pH is in this range we might notice an after taste in our water, a metallic flavor. The acidic water is picking up the taste from the copper plumbing lines and bronze faucet fixtures in our homes. One way to help reduce the “metallic” taste in your drinking water is to run the faucet for 15 - 30 seconds before taking a glass of water. This flushes out the water that has been sitting in the faucets and house lines overnight or during slow use times during the day, when you are at work, school, exercising etc. Besides improving the quality of your water by filtering out any potential bacteria , algae, dirt or sand from the water, we are also buffering the water to raise the pH from the acidic range to the neutral range. You will not only see the blue-green stains disappear, but your water will not have the metallic taste. After the water has been filtered and is ready for distribution to the public, a pH buffer is added to the water (Sodium Hydroxide) that raises the pH to 7.3 to 7.5.
The buffering agent is metered and monitored very closely to insure a high quality control is maintained. The finished water now enters the distribution mains and delivers a fresh, high quality drinking water to the City of St. Helens.

Return to the Drinking Water Filtration Facility Homepage
Return to the Article about Lead in Drinking Water
